Federal cannabis testing initiatives complete national ∆8-THC surveillance and quality infrastructure
NIST’s The Cannabis Quality Assurance Program (CannaQAP) to be key enabling infrastructure for food and beverage CPG-style regulatory regime for cannabis-infused products
The recent health advisories issued by the FDA and the CDC revealed the existence of a well-developed federal infrastructure to monitor and report adverse events related to ∆8-THC on the market today. The following details appeared in the CDC Health Alert Network (HAN) Advisory issued September 14, the same day as the FDA’s Consumer Update on the same subject, ∆8-THC:
“In March 2021, the West Virginia Poison Control Center reported two cases of adverse events related to use of delta-8 THC products in adults. In both instances, individuals mistook the products containing delta-8 THC for CBD-like products. These exposures led to symptoms consistent with cannabis intoxication. The Michigan Poison Control Center also reported two cases of severe adverse events to delta-8 THC in two children who ingested a parent’s delta-8 THC-infused gummies purchased from a vape shop. Both children experienced deep sedation and slowed breathing with initial increased heart rate progressing to slowed heart rate and decreased blood pressure. The children were admitted to the intensive care unit for further monitoring and oxygen supplementation.”
The CDC knew of the West Virginia and Michigan adverse events thanks to the inclusion of a product code specific to ∆8-THC in the American Association of Poison Control Centers (AAPCC) National Poison Data System (NPDS). More from the CDC Health Advisory:
“From January 1 to July 31, 2021, 660 delta-8 THC exposures were recorded with the new product code, and one additional case was recoded as a delta-8 THC exposure from October 2020. Eighteen percent of exposures (119 of 661 cases) required hospitalization, and 39% (258 of 661 cases) involved pediatric patients less than 18 years of age.”
In addition to the AAPCC’s NPDS,
“Syndromic surveillance data from emergency departments participating in the CDC’s National Syndromic Surveillance Program (NSSP) show an increase in visits with a mention of delta-8 THC or some variation in the chief complaint text in recent months. More than 4,400 active emergency facilities that represent portions of 49 states and Washington, DC contribute data to NSSP, accounting for approximately 71% of all U.S. non-federal emergency departments. The first suspected visit associated with delta-8 THC in NSSP was observed in September 2020, with three additional visits observed through the end of 2020. Suspected visits have generally increased monthly in 2021 (three suspected visits were observed in January; six in February; 16 in March; 11 in April; 29 in May; 32 in June; and 48 in July 2021). The majority of these visits (73%, 109 of 149 visits) occurred in the Department of Health and Human Services’ Regions 4 and 6, which are composed primarily of Southern states that have not passed state laws to allow non-medical adult cannabis use. These numbers are likely an underestimate due to the potential for inaccurate and incomplete information about products used by consumers.”
Long story short: The numbers of adverse events is still relatively low but seem to be rising as ∆8-THC has become more popular. It’s also notable that 73% of the ED visits occurred in Southern states that do not allow adult use. This suggests legal ∆9-THC and regulatory actions to test hemp-derived ∆8-THC in adult use states could be the elements of a broader strategy that avoids a federal ban on ∆8-THC, and encourages production of hemp-derived THC via alternatives to the solution chemistry SOTA.
The federal effort to ensure that cannabis products are accurately tested and labeled for CBD, THC and dozens of other cannabinoids is necessary though not sufficient to transition ∆8-THC production from the solution chemistry SOTA to a cGMP-certifiable method based on catalysis chemistry. Nonetheless, the existence of CannaQAP puts the concerns highlighted in a recent C&EN article regarding the testing and safety of ∆8-THC produced using the current solution chemistry SOTA process.
The federal effort is being led by the National Institute of Standards and Technology (NIST), part of the US Department of Commerce. The agency announced The Cannabis Quality Assurance Program (CannaQAP) in the summer of 2020. CannaQAP is itself part of an ongoing effort by NIST to encourage standardized analysis of a wide range of cannabis compounds and contaminants across an ever-expanding class of legal products.
While the immediate focus of the Cannabis Quality Assurance (CannaQAP) program will be on hemp-derived oils like CBD, officials said it’s possible they will expand the program to test marijuana flower, concentrates and edibles—a notable step for a federal agency while the intoxicating version of cannabis remains prohibited.
The program is meant to “help laboratories accurately measure key chemical compounds in marijuana, hemp and other cannabis products including oils, edibles, tinctures and balms,” NIST said in a press release. “The program aims to increase accuracy in product labeling and help forensic laboratories distinguish between hemp, which is legal in all states, and marijuana, which is not.” NIST says many labs don’t have sufficient experience conducting those tests, leading to “unreliable” results. Though cutting-edge labs today routinely offer COAs that include 20+ cannabinoids, the majority lacks the expertise and access to validated analytic standards to do so.
NIST guides standardized testing and measurement in other industries, such as dietary supplements and food safety. CannaQAP is NIST’s gambit to establish the same testing and measurement standards that underpin food and beverage CPG safety regulations.
“NIST is also planning to conduct future exercises with ground hemp and possibly marijuana,” the agency said. “Those exercises will involve measuring a larger number of compounds, including terpenes—the chemicals that give different strains of marijuana their distinct aromas—and compounds that people don’t want in their cannabis such as fungal toxins, pesticides and heavy metals. Future exercises may also include extracts, concentrates, distillates and edibles.”
Finally, NIST said it will be developing a standard hemp reference material, or “a material that comes with known, accurate measurement values” that labs can use to validate their testing methods.
“Labs can accurately measure how much sugar is in your orange juice because they have standardized methods and reference materials for that type of product,” Susan Audino, a chemistry consultant and science adviser the AOAC International, a group that creates standard methods for laboratory analysis.”
The CannaQAP program is organized (initially) around three exercises. The first exercise – the subject of the new report released last week – evaluated methods used by participating labs to determine the concentration of cannabinoids in hemp oils. Participating labs were sent two samples of hemp oils containing known concentrations of THC, CBD and 15 other cannabinoids. After testing the samples, the labs returned their results to NIST along with an explanation of their testing methods.
The primary goal of the first exercise is to show how much variability exists between testing labs and methods. 116 labs participated. Overall, 116 laboratories participated, The group consisted of a mix of commercial labs that already focus on cannabis testing, commercial or academic chemical testing labs, law enforcement agencies and assorted others. Overall, about 83 percent returned data to NIST.
The report showed “very similar results to what we’d expect in any of the similar programs that we’ve done. There’s always going to be a large amount of variability at first [but] as things progress, we expect to see that variability shrink,” according to NIST research scientist Brian Miller. “We generally don’t go into the details about the capabilities of an industry,” he added, explaining that job is better left to the USDA or state regulators. “Our main goal is just to try to improve the measurement capabilities.”
The report summarized observations of the performance of participating labs in identifying concentrations of ∆9-THC in the provided samples:
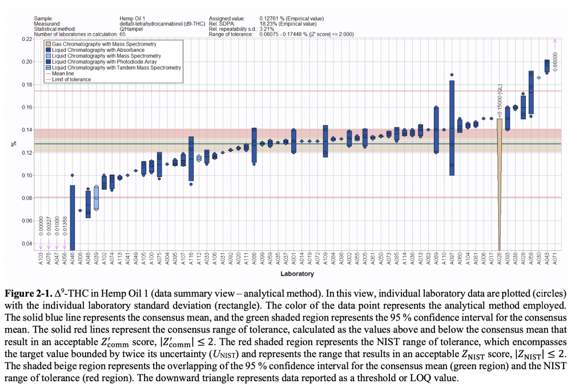
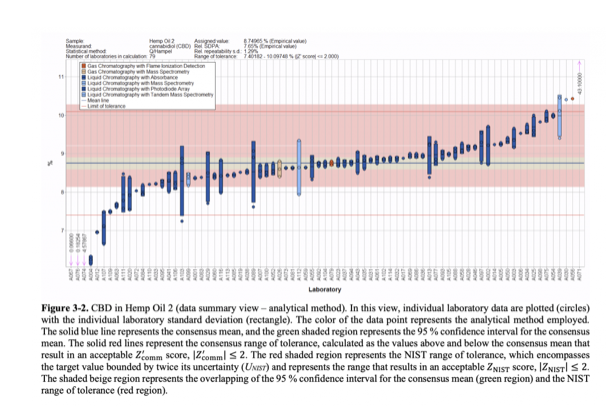
The second of CannaQAP’s exercises will focus on cannabis plant material and examine not only cannabinoids but also testing for moisture and toxic contaminants such as heavy metals. Exercise three has yet to be announced.
As part of its overall cannabis testing program, NIST is also working to develop a hemp reference material with known concentrations of various compounds, which the agency says labs could use to validate their testing methods. Currently no such cannabis reference material exists.
As the program evolves, future studies will focus on products that cannabinoids are added to, including edibles, topicals and a range of nutritional supplements.