∆8-THC testing essential for consumer safety, terrible for ∆8-THC manufacturers
Most ∆8-THC produced today would not pass minimum testing standards. Neither does the current state-of-the-art solution chemistry
A recent article in Chemical & Engineering News provided a rare view of what the community of professional chemists and chemical engineers think of cannabis chemistry. C&EN is a weekly magazine published by the American Chemical Society since 1923, providing professional and technical news and analysis in the fields of chemistry and chemical engineering.
When the house journal of one of the most august professional scientific and technical societies in the country devotes its cover to an article about the current state-of-the-art for producing an unfamiliar – to most professional chemists and chemical engineers, presumably – variant of THC, the tiny minority of ACS members who have spent some or a significant part of their professional lives in the cannabis industry would have been justified in expecting a typical C&EN treatment of the subject: Objective presentation of the need for widespread testing of ∆8-THC; thorough overview of existing and emerging methods for producing ∆8-THC; and, informed predictions of the likely evolution of ∆8-THC production methods.
The article first suggests the solution chemistry SOTA for producing ∆8-THC will yield reaction product that is toxic and could cause acute and chronic adverse effects, cases of which were tabulated and analyzed in health alerts issued by the FDA and CDC (enumerated in an earlier post):
“∆8-THC is typically synthesized from cannabidiol (CBD) extracted from hemp. The reaction often yields a high percentage of ∆8-THC, as well small amounts of other cannabinoids and reaction by-products. Little is known about the health effects of these impurities, and chemists have not identified all of them.”
“The conversion of CBD to ∆8-THC involves refluxing CBD in an organic solvent, such as toluene or heptane, with p-toluenesulfonic acid or another acid that serves as a catalyst. The reaction is typically run for 60–90 min.”
C&EN
“[It’s possible that one day there will be cannabis plants that contain sufficient ∆8-THC to extract in pure form. But for now, cannabis plants typically contain 0.1% ∆8-THC or less]. We have seen reports of plants containing as much as 1%, [but those are exceptions. Economical extraction of ∆8-THC from cannabis requires levels of about 15–20%]. Genetics folks are going after that now, [but synthetic products will dominate for a while].”
Jeffrey Raber Cofounder and CEO Werc Shop (CA)
In 2018, Raber’s Werc Shop released a white paper that included HPLC traces of clean and dirty batches of ∆8-THC. From the white paper: “Often Delta-8 is present alongside Delta-9 and various unidentified cannabinoid analogues as depicted in the HPLC chromatogram below. These products appear to be the result of uncontrolled processing steps and often have attendant side-products and unidentified cannabinoid derivatives present.”
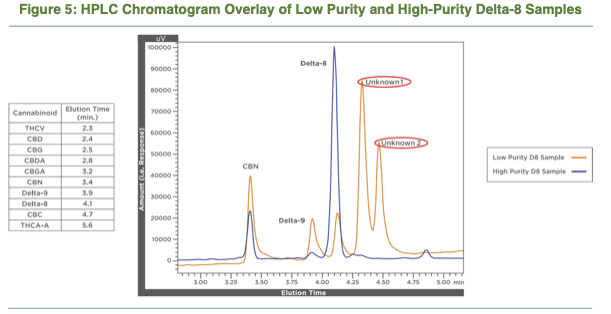
https://thewercshop.com/wp-content/uploads/2018/09/TWS-Delta-8-Whitepaper-Sept-2018.pdf
The Werc Shop’s findings have since been confirmed by multiple outlets. For example, a San Diego-based testing lab noted earlier this year:
“The process of creating ∆8-THC from cannabidiol isolate is extremely difficult, and it’s nearly impossible to completely separate the ∆8-THC from ∆9-THC through distillation. The only companies we’ve seen have success with this conversion take additional chromatography steps after distillation to remediate the ∆9-THC from the batch of product. [Less than one percent of the 2,000 samples tested last year came back as pure ∆8-THC, and most contained more ∆9-THC than reported. Some of the samples came back] looking like a rain forest. We don’t even know what’s in there. It’s a mess of chromatography that would take a half-million dollars in testing to uncover.”
Josh Swider PhD Founder and CEO InfiniteCAL (CA)
C&EN quotes industry players in ∆8-THC testing and production to connect aspects of the current SOTA to potential reaction hazards and reaction product toxicity:
“These are pretty aggressive synthetic conditions that use strong acids. They might be using strong bases to neutralize. They can use metal catalysts. I hear different people doing it different ways.”
“I believe that ∆8-THC has a legitimate place in therapeutics and potentially adult use, but I just don’t see anybody doing it appropriately. It’s all bathtub gin.”
Christopher Hudalla President Chief Scientific Officer ProVerde Laboratories (MA, ME)
“Most people are not actually taking the time to distill it or use chromatography [to separate ∆8-THC from unwanted reaction leftovers or by-products]. A lot of irresponsible production is going on in the sense that most of these people are getting their information from online forums, and many of them aren’t necessarily trained chemists.”
Kyle Boyar Staff Research Associate UCSD Center for Medicinal Cannabis Research
“The conversion of CBD to ∆8-THC is an exothermic reaction, so it creates a lot of heat. This needs to be done in a controlled environment [such as under dry ice and acetone]. An ice bath isn’t cold enough. People who tried that approach blew stuff up. [Popular solvents like dichloromethane] should not be used without appropriate ventilation and controls because it’s a silent killer. A lot of these shops, even the shops in the legal markets, are not ready for this kind of activity”
“I am trying to make sure the science is good. I’m working with peers all over the country and looking at different purification methods.”
Tiffany Coleman, Director of Quality and Processing Carbidex (MI)
The same industry participants argue that ∆8-THC produced using current methods is likely be harmful to consumers:
“My concern is that we have no idea what these products are. Consumers are being used as guinea pigs. To me, that’s horrific.”
“So far, I have not seen one that I would consider a legitimate ∆8-THC product. There’s some ∆8-THC in there, but there’s very frequently up to 30 [chromatographic] peaks that I can’t identify.”
Christopher Hudalla President Chief Scientific Officer ProVerde Laboratories (MA, ME)
C&EN concludes that as long as ∆8-THC is made using the current solution chemistry SOTA, testing ∆8-THC will be impossible:
“But existing independent analytical labs can’t handle the burden of exhaustive testing on all ∆8-THC products, according to Amber Wise, scientific director at Medicine Creek Analytics, a cannabis testing firm in Washington State. There are a handful of methods being discussed on online forums that use chemicals ‘that I would not want to have as residuals’ in ∆8-THC products, such as dichloromethane and trichloroacetic acid, Wise says. Her lab hasn’t developed methods to test for those chemicals, she says, adding that it’s not practical to develop methods for every possible reagent people are using to make ∆8-THC. Instead, Wise says, regulators should require manufacturers to reveal what chemicals they use to make ∆8-THC and what compounds are in their final products.”
The article’s concerns regarding the state-of-the-art are valid. However, the accompanying historical assessment is improperly contextualized and in certain cases, wrong. Facile isomerization of CBD to THC, catalyzed by acids and metal salts, was discovered decades before the 1960s as the author noted. A process was described in a seminal paper by a pioneer of cannabinoid research, Roger Adams (JACS, 1940). Due in large part to persistent historical mischaracterization and active stigmatization of cannabis by the scientific community, CBD-to-THC conversion research has been confined to a small cohort in academia until the last decade, when the emergence of CBD as a major commercial cannabinoid drove explosive growth of a scientific community devoted to cannabinoid research.
Unfortunately, 80 years of mischaracterization and stigmatization have created a body of persistent misinformation – today propagated in online blogs and spread virally – a portion of which is featured in the article. For example, concerns about the exothermic nature of the catalytic CBD-to-THC conversion are presented as dispositive but are false. Implication of olivetol as a common degradation product is also without foundation.
Commercially available analytic COAs include quantification of 20-25 cannabinoids, including ∆8-THC and ∆9-THC, reaction side products, and residuals/leachates. These COAs are commonly shared by many manufacturers of cannabis-derived products, both to comply with emerging regulations and to meet growing consumer demand for the information on product labels.
Especially troubling from an accuracy standpoint, when describing the synthetic nature of CBD-derived THC, the article fails to remind the reader that ∆9-THC is not actually produced by the plants naturally but generated by the chemical process of thermal decarboxylation of THC-9-acid at the extraction facility or combustion.
The preceding aside, we could not agree more with the author that full analysis of ∆8-THC -associated degradation products as well as the establishment of their safety are crucial for end users. This safety issue must be addressed by regulators at state and federal levels before burgeoning customer demand for products containing ∆8-THC can be met. But pharmaceutical grade CBD is now widely available at <$200/kg purchased in bulk, enabling commercial-scale conversion and testing.